Cilia assembly and development
Principal investigator: Bénédicte DURAND
Cilia | Centriole | Cytoskeleton | Cell architecture | Ciliopathies | Drosophila | Microtubules | Muscle | Nesprin
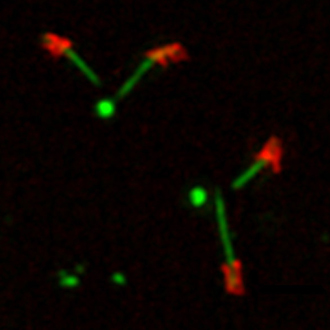
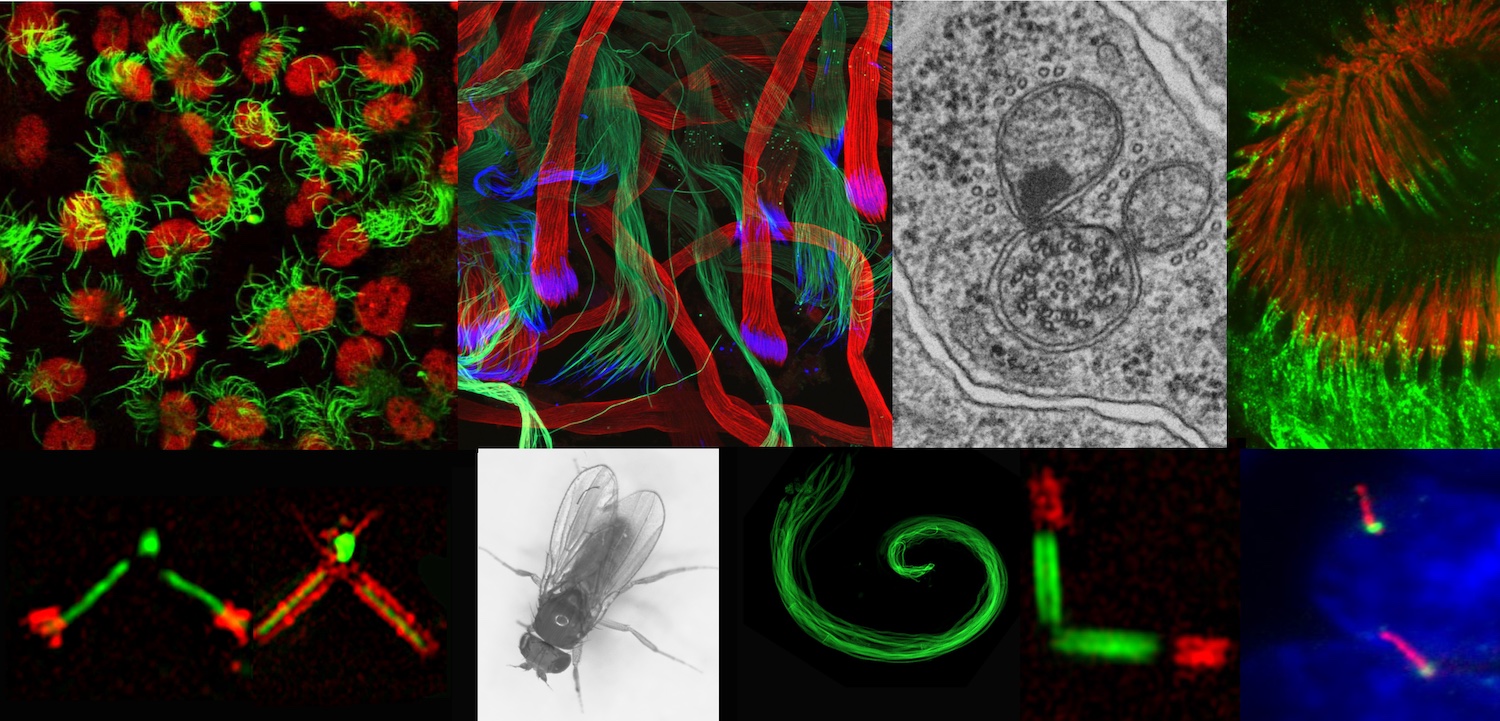
One of the key steps in cilia assembly is the conversion of the centriole to the basal body, a process strictly correlated with cell cycle progression. One of the current challenges in understanding cilia and flagellum assembly is our ability to resolve the molecular organization of this structure and to identify the role of each component of this highly organized assembly. For this purpose, high resolution microscopy approaches are developed in the team: airyscan, 3D-SIM, electron microscopy and expansion microscopy coupled with STED microscopy applied to Drosophila ciliated tissues or mammalian cells. This will help us to understand the three dimensional organization of these assemblies, but also the functional and temporal hierarchy of the constitutive elements. We combine these observations with functional genetics strategies in Drosophila (including CripsR/Cas9 genome editing) and in mammalian cell culture. In addition, we are developing biochemical approaches (proximity labeling) to understand the molecular composition of the centriolar or ciliary scaffolds. We have identified several proteins that are involved in human ciliary pathologies but also in neuronal degeneration (amyotrophic lateral sclerosis) and cardiac muscle pathologies (cardiomyopathy). We aim to understand how these proteins contribute to the biogenesis of cilia and centrioles and may be at the origin of these different pathologies.
For the general public
Cilia and flagella are small cellular protrusions at the cell surface that play various roles: they can be motile and propel cells (spermatozoa) and fluids (respiratory mucus), or they can be immotile and serve as cellular antennae capturing extracellular signals, allowing cells to respond and adapt to their environment. Abnormalities in cilia formation or function are responsible for many rare and often severe hereditary diseases, grouped under the term ciliopathies. More, cilia and centrioles on which they are built, are also important players in the regulation of cancerous processes because of their role in the control of cell division and cell signaling.
Our team aims to understand how cilia are assembled from centrioles and what are the mechanisms underlying their diversity of function. We are developing functional genetic, biochemical and high-resolution imaging approaches to identify and understand the function of new genes involved in cilia assembly. We mainly use the Drosophila model and mouse or human cell models. Our work has implications for the understanding of diseases associated with dysfunctions of these organelles.